The human gut microbiome consists of trillions of tiny bacteria, viruses, protozoa and microbes. The colonization of the gastrointestinal tract by micro-organisms, begins within a few hours of birth and concludes around three to four years of age. The nature of the colonic microbiota is driven by several factors such as mode of delivery (vaginal or c-section), breast feeding, geographical location, genetics, age and gender. It is a very dynamic entity influenced by environment and nutritional behaviours.
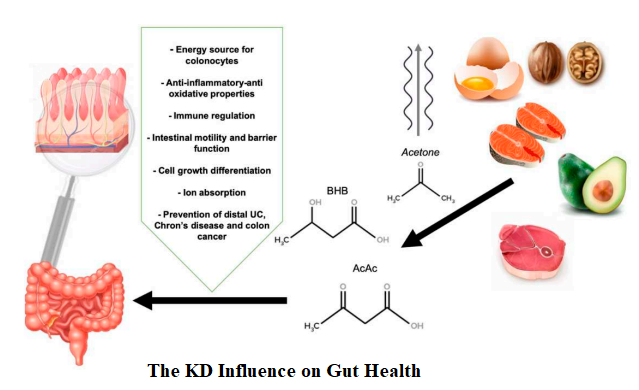
The gut microbiome is greatly influenced by diet, and, a good ecological microbiome community is connected with better health, thus offering a range of opportunity for improving our health by changing the microbiota composition through different diet patterns. These dietary patterns composed of non-refined foods and a high intake of microbiota accessible carbohydrate as represented by the walls of plant cells, cellulose, hemicelluloses, pectin and resistant starch, are capable of supporting the growth of specialist microbe producing short chain fatty acids (SCFAs) such as butyrate. SCFAs are a prominent energy source for human colonocytes and the signalling key molecules between the gut microbiota and the host. They also contribute to the regulation of the systemic immune function, to the direct appropriate immune response to pathogens and influence the resolution of inflammation.
Western diet, which is high in simple sugars and low in fibre, reduces the production of SCFAs, shifting the gastrointestinal microbiota metabolism to the production of detrimental metabolites and favouring the expansion of bacteria associated with chronic inflammation.
The ketogenic diet (KD) is a dietary protocol that has been used since the 1921 as a treatment for refractory epilepsy and is currently gaining popularity as a potential therapy for obesity and related metabolic disorders (metabolic syndrome). The KD has been a hot topic in research and an evolving area of interest has been the effect of the KD on the gut microbiome.
KD influences the gut health through increasing metabolite SCFAs produced by different microbes. SCFAs have been shown to alter chemotaxis and phagocytosis; reduce reactive oxygen species (ROS); change cell proliferation and function; have anti-inflammatory, antitumorigenic, and antimicrobial effects; and alter gut integrity. It highlights the role of SCFAs as a major player in maintenance of gut and immune homeostasis.
Effect of KD on gut microbiota related to the anti-seizure effect:
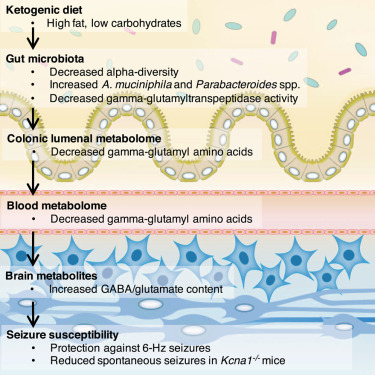
- A study conducted on mice by Olson et al, 2018 showed that two species of bacteria, Akkermansia and Parabacteriodes were significantly increased when fed ketogenic diets and gnotobiotic colonization with these microorganisms revealed an anti-seizure effect in germ-free mice or treated with antibiotics.
- The increase of these two bacteria species in the gut led to a decreased production of γ-glutamyl transpeptidase by the gut microbiome, the enzyme catalyzes the transfer of γ-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid forming glutamate.
- Moreover, they observed a decrease in subset of ketogenic γ-glutatamylated (GG) amino acids (i.e., γ-glutamyl-leucine) both in the gut and blood. This fact, in turn, had the effect of increasing the ratio of GABA to glutamate in the brain of mice.
- The researchers suggested that KD-microbiota-related limitation in GG amino acids plays a pivotal role on anti-seizure effect, confirmed by the previous studies showing GGT activity to modify the electrical activity of seizure.
There is evidence that the microbiota in drug-sensitive epilepsy (DSE) and drug-resistant epilepsy (DRE) is different. A study by Peng et al, 2018 showed that patients with DRE harbored an altered composition of the gut microbiome characterized by a significantly increased abundance of some rare bacteria. There was an abnormal increase in numerous rare bacteria mainly belonging to the phylum Firmicutes. Increased Firmicutes is also seen in many other diseases including autism spectrum disorders and parkinson’s disease. Specifically, the phylum Verrucomicrobia was significantly increased in patients with DRE. Over-expression of Verrucomicrobia was also found in patients with periodontal diseases and multiple sclerosis. It was speculated that gut dysbiosis may be involved in the pathogenesis of DRE. The study also observed the gut microbiota in patients with DSE was similar to that of healthy controls.
A study in epileptic children by Xie et al, 2017, found a higher amount of pathogenic proteobacteria (Escherichia, Salmonella and Vibrio) and Firmicutes, which significantly decreased after KD treatment and an increase of Bacterioidetes both in healthy subjects and in patients. Bacteroides spp. are strictly connected with the digestion and metabolism of high-fat nutrients and the regulation of the secretion of 6–17 interleukins in dendritic cells, which is connected with the seizure effects on epileptic patients.
A review by Thambi et al, 2020 found that alteration in the gut microbiota composition may be a potential indicator of responsiveness/resistance to a KD in epilepsy. However, there is need for well-designed randomized case-control and cohort human studies.
Effect of KD on gut microbiota related to other diseases:
Gut microbiome plays an important role as a separate endocrine organ that maintains host energy homeostasis and stimulates host immunity. Gut dysbiosis has been recognized as a key factor driving metabolic diseases such as diabetes, atherosclerotic cardiovascular disease, non-alcoholic fatty-liver disease, Alzheimer’s disease, and certain obesity-related cancers.
Alzheimer’s Disease:
Ma et al, 2018 observed that KD fed mice had a variety of neurovascular improvement strictly linked to a lower risk of developing Alzheimer’s disease. These beneficial effects may be connected with the changing on gut microbiota composition, more specifically with the growing quota of beneficial bacteria, Akkermansia Muciniphila and Lactobacillus, which have the ability of generating SCFAs. They found a reduction in pro-inflammatory microbes such as Desulfovibrio and Turicibacter.
Autism:
A 2016 study investigated whether or not a KD could ensure benefits in the gut microbiome in murine model of autism. They concluded that the KD had an “anti-microbial” effect by decreasing the overall richness of microorganisms both in cecal and fecal matter, and as well as improved the ratio of Firmicutes to Bacteroides species thereby improving ability of KD to mitigate some of the neurological symptoms associated with ASD (Newell et al, 2016).
Other Disorders:
A study in children given KD, found a reduction of Bifidobacteria, as well as E. rectale and Dialister, which are correlated with health promoting benefits such as the prevention of colorectal cancer, IBS and necrotizing entercolitis. Researcher identified a relative abundance of Actinobacteriaand Escherichia coli that may be due to the KD restricted on carbohydrate (O’Callaghan et al, 2016).
Factors Affecting Microbiota during KD: What Should We Consider?
- Artificial sweeteners:
- Several evidences demonstrated that artificial sweeteners have a negative impact on both host and gut health.
- Nettleton et al, 2016 found that low calorie sweeteners, such as acesulfame potassium (Ace-K) and sucralose, disrupted the structure and function of gut microbiota and gut mucosa.
- Qiao-Ping Wang, 2018 conducted a study which revealed that artificial sweeteners has bacteriostatic effects and as well as change the composition of microbiota.
- These findings, according to the fact that the routine consumption of artificial sweeteners may increase the risk of cardiometabolic diseases, suggested that these chemical substitutes may be detrimental for human health and should be avoided.
- The uses of stevia, monkfruit and erythritol have been widely adopted as safe natural sweeteners.
- Pre and Probiotics:
- The major source of prebiotics is represented by fructo-oligosaccharides, inulin, lactulose galacto-oligosaccharides and trans-galacto-oligosaccharides.
- Fermentation of prebiotics by gut microbiota produces SCFAs, which positively modulate the composition of microbiota (by increasing intestinal bifidobacteria and lactic acid bacteria), providing an energy source for colonocytes.
- Probiotics are living bacteria (especially from the genera Bifidobacterium and Lactobacillus) and yeasts that, when administrated in an adequate amount, show positive effect on human health.
- It has been reported that foods enriched with these microorganisms are able to aid recovery and improve gut microbiota, reaching the state of eubiosis.
- Cultured-milk products (yogurt), traditional buttermilk, fermented cheese, pickles contain several and different “friendly bacteria” such as Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgarius, Lactobacillus reuteri, Saccharomyces boulardii and Bifidobacterium bifidum.
- Fats:
- Specific types of fatty acids affect the gut microbiota in different way.
- Diets high in saturated fatty acids led to negative effects on the gut microbiota.
- Monounsaturated fats affected negatively the gut microbiota decreasing bacteria richness, while diets rich in polyunsaturated fatty acids (with opposite effects when comparing omega 3 vs. omega 6 fats) did not change richness and diversity.
- Proteins:
- Studies have demonstrated that plant-derived protein get better benefits on gut microbiome along with positive effects on the host metabolism.
- Plant-derived protein, such as mung bean protein (as a part of high fat diet), increased Bacteroidetes while decreasing Firmicutes as well as pea protein increased strains of Bifidobacterium and lactobacillus.
In conclusion, ketogenic diet has demonstrated to be a powerful tool in modulating and reshaping gut microbiota.
References:
- Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes (Basel). 2019, 534.
- Olson C.A, Vuong H.E, Yano J.M, Liang Q.Y, Nusbaum D.J, Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 174, 497.
- Peng Anjiao, Qiu Xiangmiao Lai, Wanlin Li, Wanling Zhang, Lin Zhu, Xi He, Shixu Duan, Jianan Chen, Lei. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Research. 2018,147, 102–107.
- Xie G, Zhou Q, Qiu C.Z, Dai W.K, Wang H.P, Li Y.H, Liao J.X, Lu X.G, Lin S.F, Ye J.H, et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017,23, 6164–6171.
- Thambi M, Nathan J, Radhakrishnan K. Can change in gut microbiota composition be used as a surrogate marker of treatment efficacy of ketogenic diet in patients with drug-resistant epilepsy? Epilepsy Behav. 2020,113:107444
- Ma D, Wang A.C, Parikh I, Green S.J, Hoffman J.D, Chlipala G, Murphy M.P, Sokola B.S, Bauer B, Hartz A.M.S, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018,8:6670.
- Newell C, Bomhof M.R, Reimer R.A, Hittel D.S, Rho J.M, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism. 2016,7:37.
- O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016,7:925.
- Nettleton J.E, Reimer R.A, Shearer J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164, 488–493.
- Wang Q.P, Browman D, Herzog H, Neely G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080.
